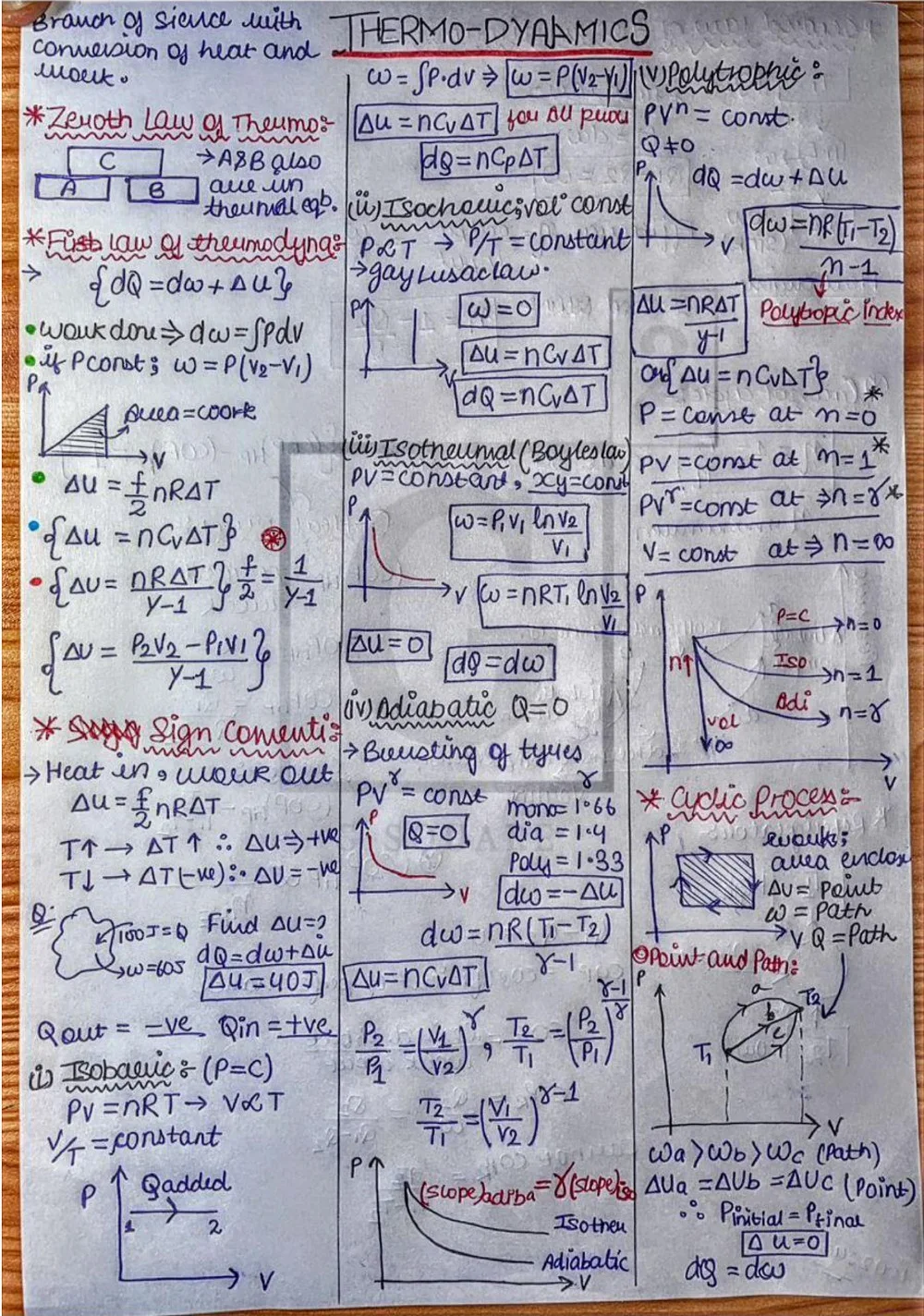
Thermodynamics is a fascinating branch of physics that delves into the relationship between heat, temperature, and work, and how these factors influence energy transfer and transformation within a system. This field of study plays a crucial role in various scientific and engineering disciplines, forming the foundation for understanding heat engines, refrigerators, and even the workings of stars.
What is Thermodynamics?
In simpler terms, thermodynamics is concerned with the macroscopic behavior of matter, focusing on the bulk properties of a system rather than the microscopic details of individual atoms or molecules. It establishes principles governing the transfer and transformation of thermal energy (heat) between a system and its surroundings. These principles, known as the laws of thermodynamics, provide a framework for predicting the direction and efficiency of energy conversions.
Types of Thermodynamic Systems and Processes
In thermodynamics, a system is a specific region of matter that we're interested in studying. The surrounding is everything outside the system that interacts with it. Systems can be categorized as open, closed, or isolated depending on their ability to exchange matter and energy with the surroundings.
Thermodynamic processes describe the changes that occur within a system. Some important types of processes include:
⦿ Isothermal process: Temperature remains constant (T = constant).
⦿ Adiabatic process: No heat transfer occurs between the system and the surroundings (Q = 0).
⦿ Isobaric process: Pressure remains constant (P = constant).
⦿ Isochoric process: Volume remains constant (V = constant).
Examples of Thermodynamics in Action
The applications of thermodynamics are vast and encompass many aspects of our daily lives. Here are a few examples:
⦿ Heat engines: Car engines, power plants, and jet engines all function based on thermodynamic principles, converting heat energy into mechanical work.
⦿ Refrigerators and air conditioners: These appliances operate on a reversed Carnot cycle, using work input to extract heat from a cold reservoir and transfer it to a hot reservoir, achieving a cooling effect.
⦿ Chemical reactions: Thermodynamics helps predict the spontaneity and feasibility of chemical reactions by analyzing their enthalpy and entropy changes.
Key Formulas in Thermodynamics
Here's a quick reference table containing some essential formulas used in thermodynamics:
⦿ First Law of Thermodynamics: ΔU = Q + W (Change in internal energy = Heat transfer + Work done)
⦿ Work done in an isothermal process: W = nRT ln(V2/V1) (where n is the number of moles, R is the gas constant, T is temperature, V1 and V2 are initial and final volumes)
⦿ Work done in an adiabatic process: W = (P1V1 γ - P2V2 γ) / (γ - 1) (where γ is the specific heat ratio, P1, V1 and P2, V2 are initial and final pressure and volume)
Remember, this is just a basic overview to get you started with thermodynamics. As you delve deeper into this subject, you'll encounter more complex concepts and a wider range of formulas to explore.
Frequently Asked Questions about Thermodynamics
1. What's the difference between heat and temperature?
Heat is the transfer of thermal energy between objects at different temperatures. It's a process, like the flow of a river. Temperature, on the other hand, is a measure of the hotness or coldness of an object. It's like the water level in the river, indicating the intensity of thermal energy.
2. Can heat flow from a cold object to a hot object?
No, according to the second law of thermodynamics (not covered in detail in Class 11), heat naturally flows from a hotter object to a colder object, tending towards a state of increased entropy (disorder).
3. What's the significance of the sign convention in the first law of thermodynamics?
The sign convention matters! A positive Q indicates heat entering the system, while a negative Q signifies heat leaving the system. Similarly, positive W implies work done by the system on the surroundings, and negative W represents work done on the system by the surroundings.
4. Are there any real-life examples of isothermal processes?
While achieving a perfectly isothermal process is challenging, slow and controlled expansion or compression of a gas can be a good approximation. Imagine a well-insulated piston slowly compressing air; the process can be considered nearly isothermal.
5. Why are adiabatic processes important?
Adiabatic processes are crucial in understanding rapid changes in pressure and volume. For instance, the rapid compression of air in a car engine during combustion is an adiabatic process. Understanding these processes helps engineers design efficient engines.
6. Where can I find practice problems to solidify my understanding of thermodynamics?
Many physics textbooks and online resources offer practice problems related to thermodynamics. Utilize your textbook's problem sets, explore resources from Khan Academy or Physics Classroom websites, or consult online tutoring platforms for additional practice.