Heat is a fundamental concept in physics that governs the flow of thermal energy between objects. It plays a crucial role in various natural phenomena and technological applications. This article delves into the world of heat in the context of Class 11 Physics, exploring its definition, different types, real-world examples, and practical applications. We will also provide a table summarizing key formulas related to heat transfer.
Understanding Heat: A Transfer of Thermal Energy
Heat is not a substance itself, but rather the transfer of thermal energy between objects at different temperatures. This transfer occurs due to the inherent tendency of heat to flow from a hotter object (higher temperature) to a colder object (lower temperature) until thermal equilibrium is reached, where both objects possess the same temperature.
Types of Heat Transfer: Conduction, Convection, and Radiation
Heat transfer can occur through three primary mechanisms:
⦿ Conduction: This method involves the direct contact between objects. When two objects at different temperatures are in contact, thermal energy flows from the hotter object to the colder one through the microscopic vibrations of their atoms. Examples of conduction include heating a metal spoon in hot soup or feeling warmth from a radiator.
⦿ Convection: This process involves the transfer of heat through the movement of fluids (liquids or gases). As a fluid is heated, it expands and becomes less dense, rising upwards. Cooler, denser fluid then sinks, creating a continuous circulation pattern that transports heat. Convection currents are responsible for phenomena like air circulation in a heated room or the movement of water in a boiling pot.
⦿ Radiation: Unlike conduction and convection, radiation does not require any medium for heat transfer. It involves the transfer of thermal energy in the form of electromagnetic waves. The sun's heat reaching Earth is a prime example of radiant heat transfer. Even hot objects like the filament in a light bulb emit infrared radiation, which we perceive as heat.
Examples of Heat in Action
Heat plays a vital role in numerous everyday processes and technological advancements:
⦿ Cooking: When we apply heat to food, it cooks by transferring thermal energy to the food, causing its molecules to vibrate faster and ultimately changing its state (e.g., from raw to cooked).
⦿ Power Generation: Thermal power plants utilize heat generated from burning fossil fuels or nuclear reactions to convert water into steam, which drives turbines and produces electricity.
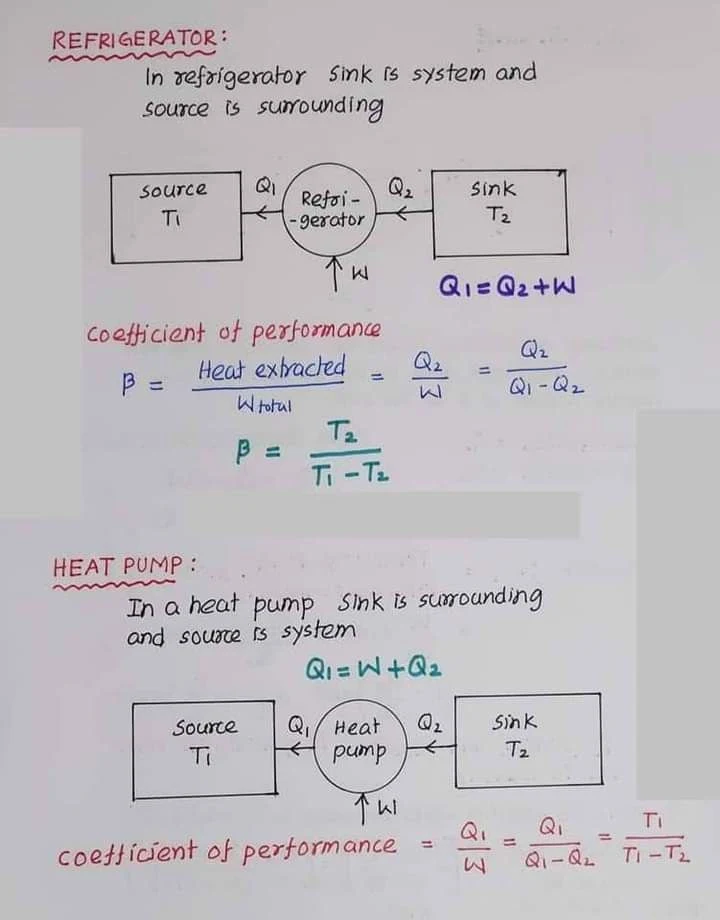
⦿ Refrigeration: Refrigerators work by removing heat from a designated space (the inside of the fridge) using a refrigerant. This heat is then transferred to the surrounding environment (the room) through the back of the refrigerator.
Formula Table for Heat Transfer Calculations
Here's a table summarizing some key formulas used in heat transfer calculations:
⦿ Heat Capacity (C): C = Q / ΔT (C: heat capacity in J/kg°C, Q: heat transferred in Joules, ΔT: change in temperature in °C)
⦿ Specific Heat Capacity (c): c = Q / (mΔT) (c: specific heat capacity in J/kg°C, Q: heat transferred in Joules, m: mass in kg, ΔT: change in temperature in °C)
⦿ Thermal Conductivity (k): Q = -kAΔT / Δx (k: thermal conductivity in W/mK, A: area in m², ΔT: temperature difference in K, Δx: distance in m)
By understanding these concepts and formulas, you can effectively analyze and solve problems related to heat transfer in various contexts.
Frequently Asked Questions about Heat
1. What's the difference between heat and temperature?
Heat is the transfer of thermal energy due to a temperature difference. Temperature, measured in Kelvin (K) or Celsius (°C), indicates the degree of hotness or coldness of an object. It's like a reading on a thermometer, whereas heat is the actual flow of energy.
2. Can heat flow from a cold object to a hot object?
No, heat naturally flows from a hotter object to a colder one until thermal equilibrium is reached. However, if external work is done (e.g., using a refrigerator), heat can be transferred against the natural tendency.
3. Which type of heat transfer is most efficient for cooking food?
Conduction is generally the most efficient mode for cooking solid food items like steaks or pancakes as it directly transfers heat through contact. Convection currents are better for liquids (soups, stews) as they ensure even heat distribution.
4. How can we minimize heat loss in a building?
Utilizing insulation materials in walls and roofs helps trap heat inside during winters and prevent excessive heat gain during summers. Additionally, sealing air leaks and using energy-efficient appliances further minimize heat loss.
5. What are some alternative sources of heat generation besides fossil fuels?
Solar energy, geothermal energy, and nuclear energy are all potential alternatives for heat generation. Solar panels capture sunlight and convert it into usable heat or electricity. Geothermal energy utilizes the Earth's internal heat, while nuclear power plants generate heat through controlled nuclear reactions.