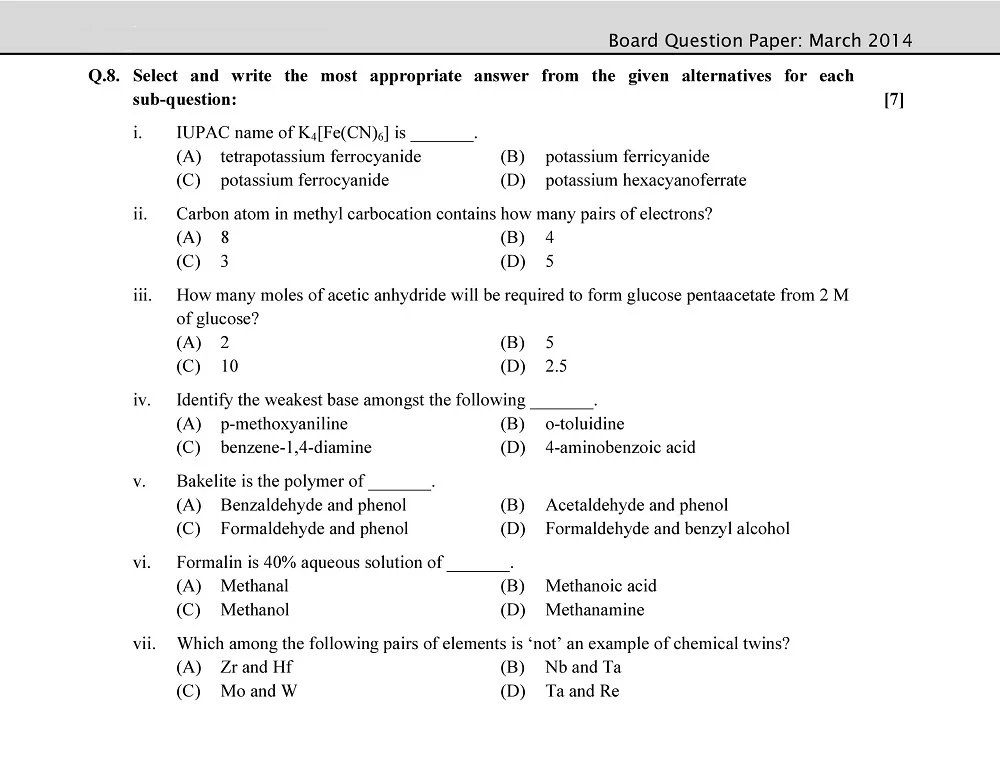
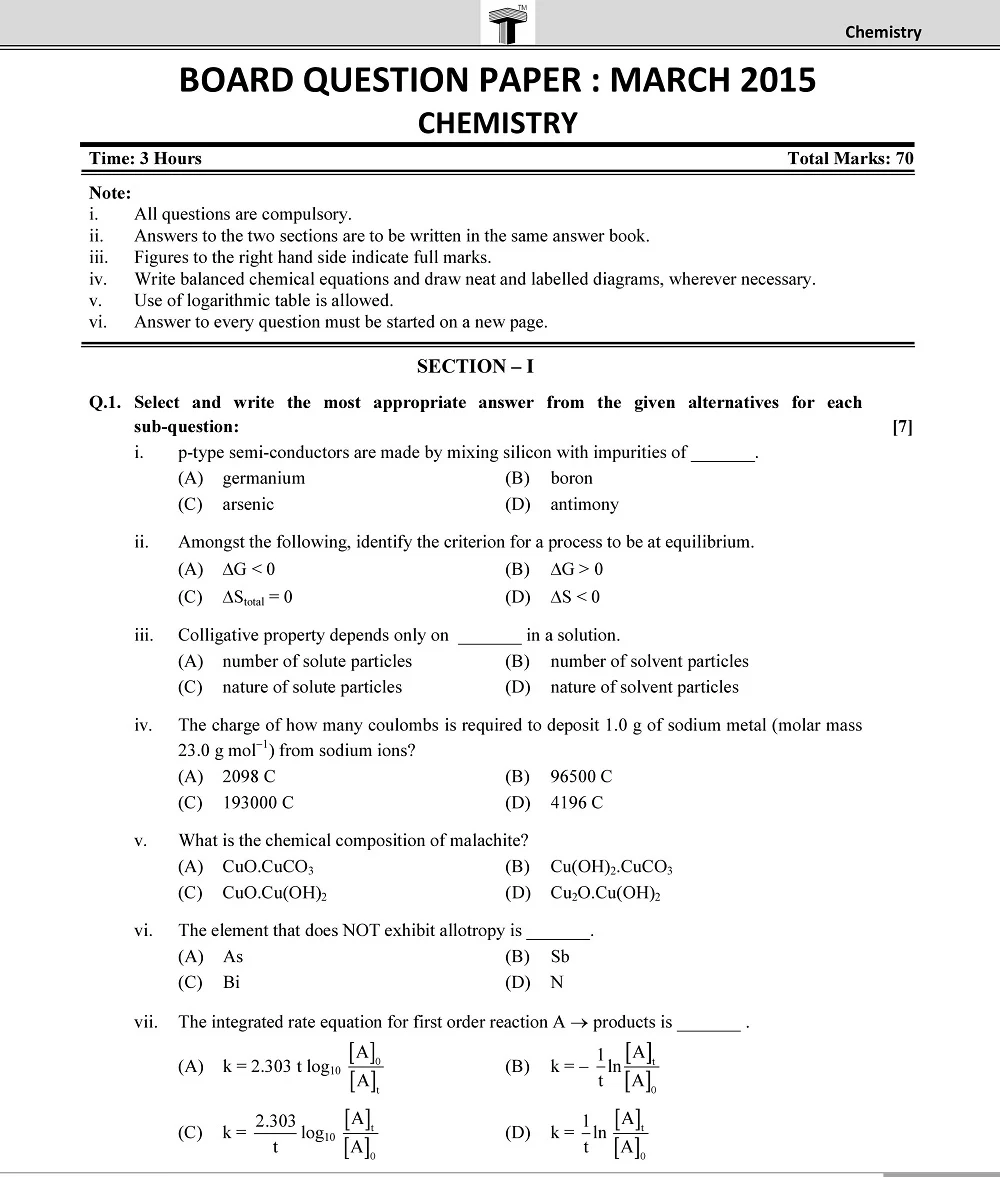
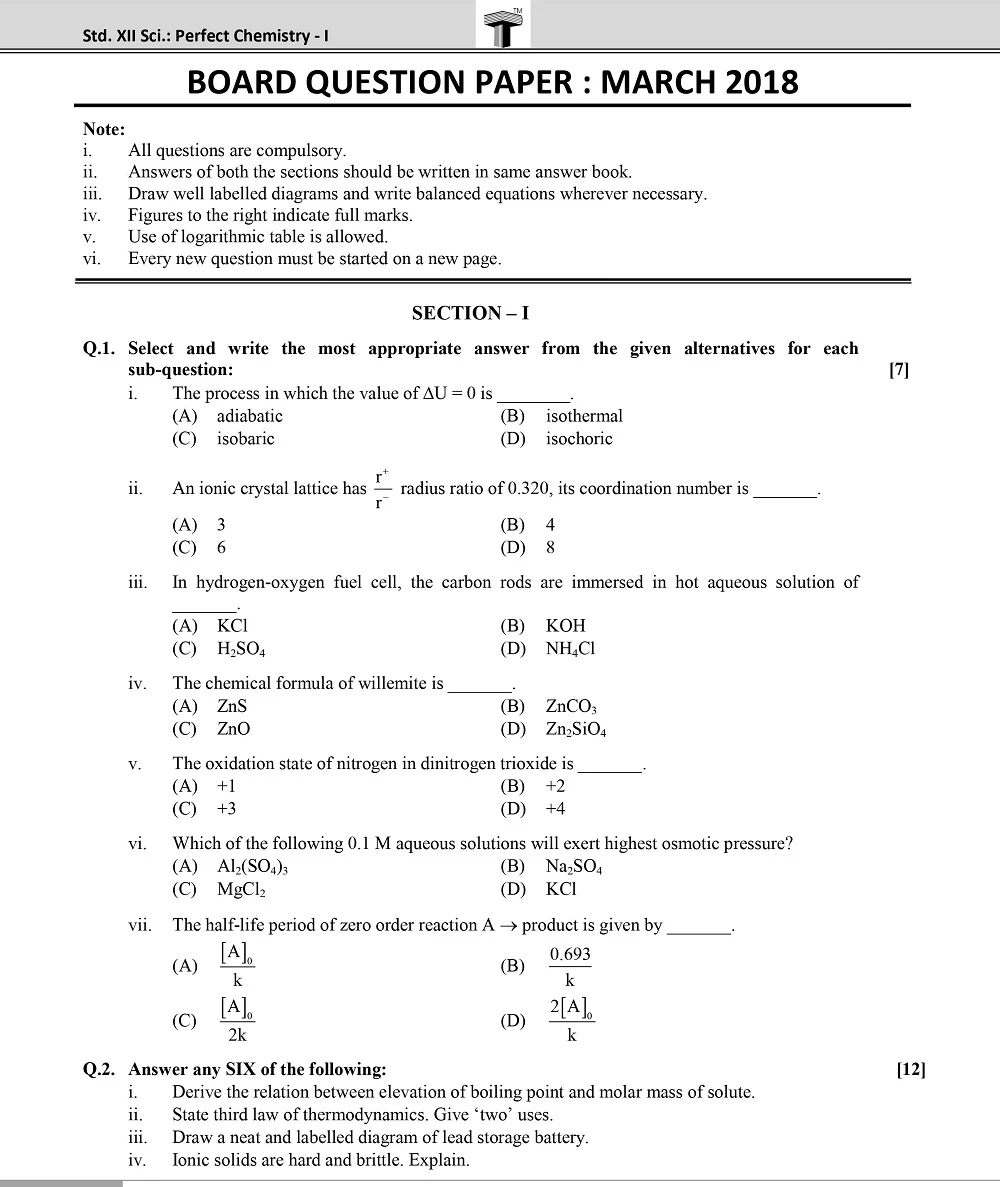
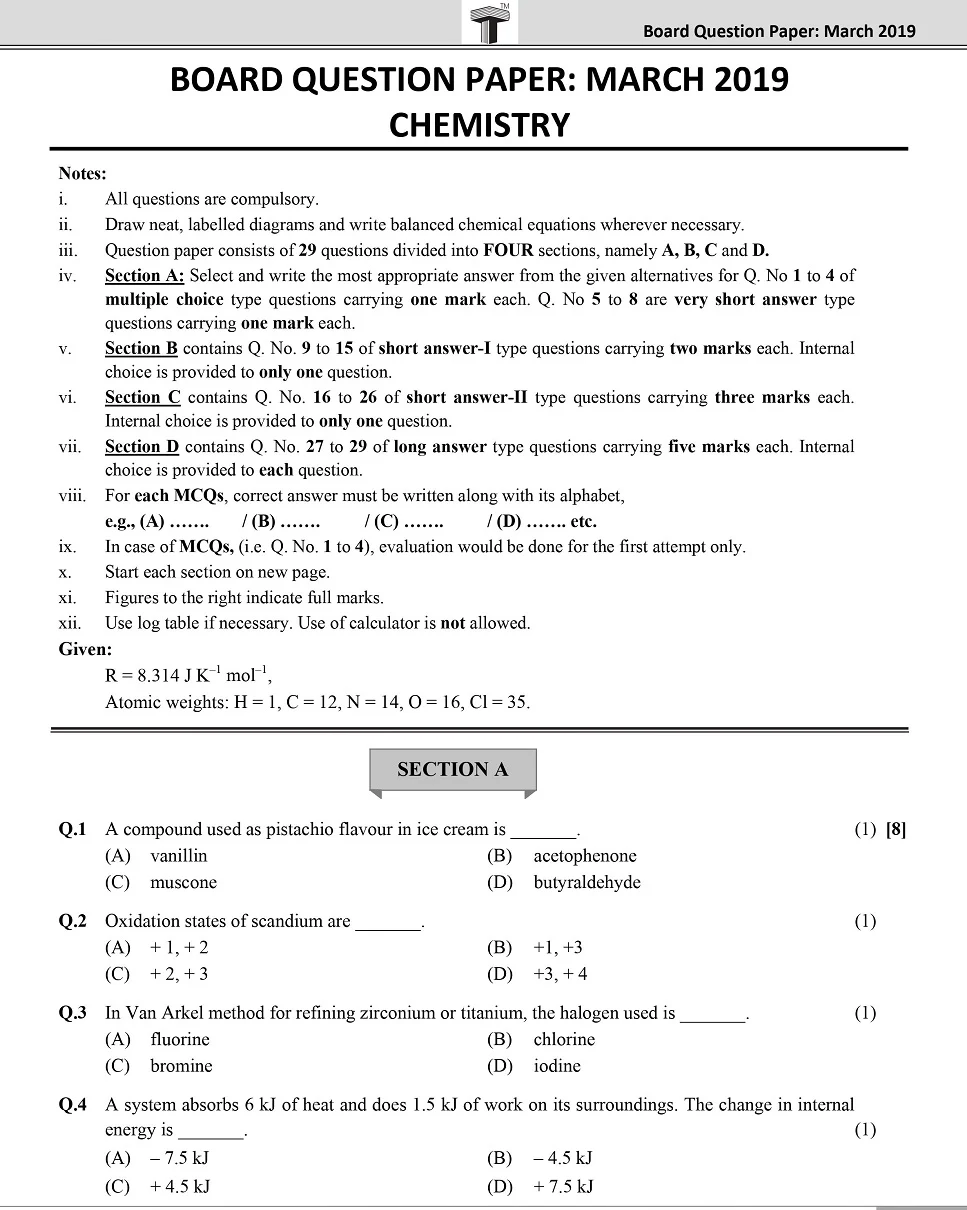
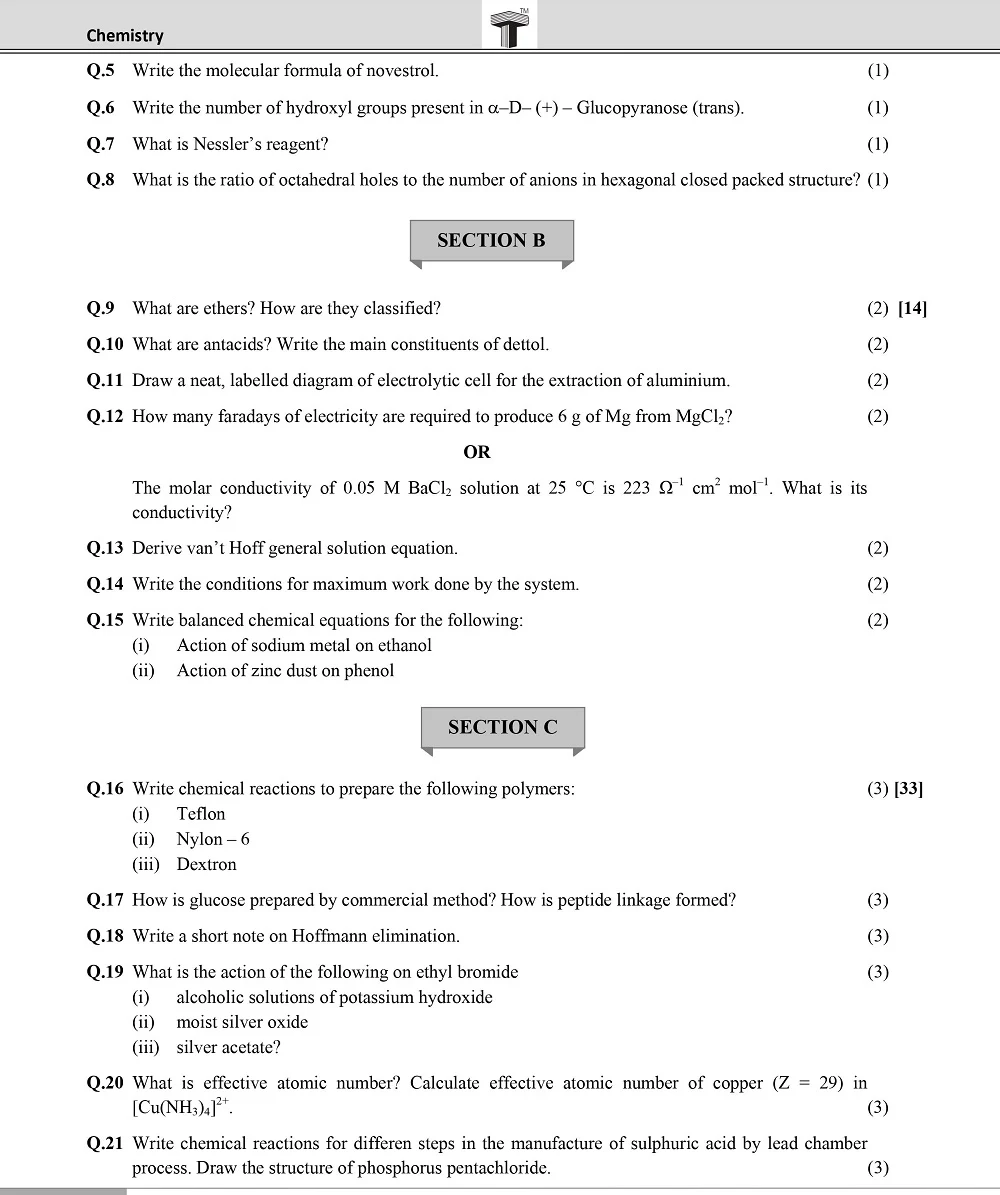
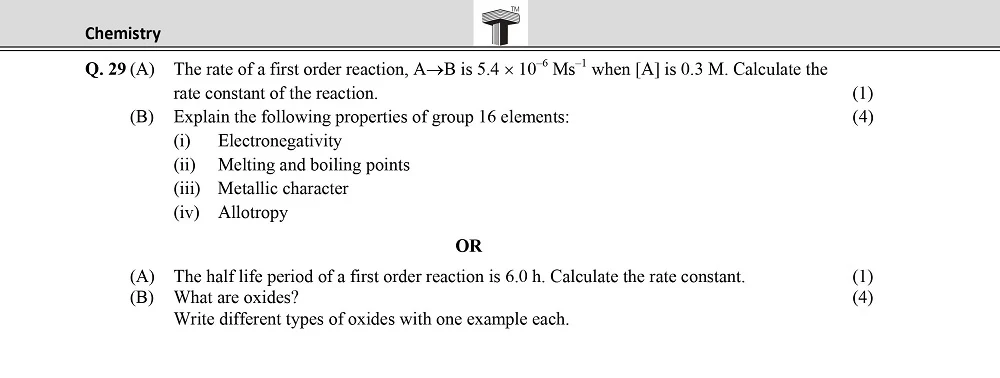
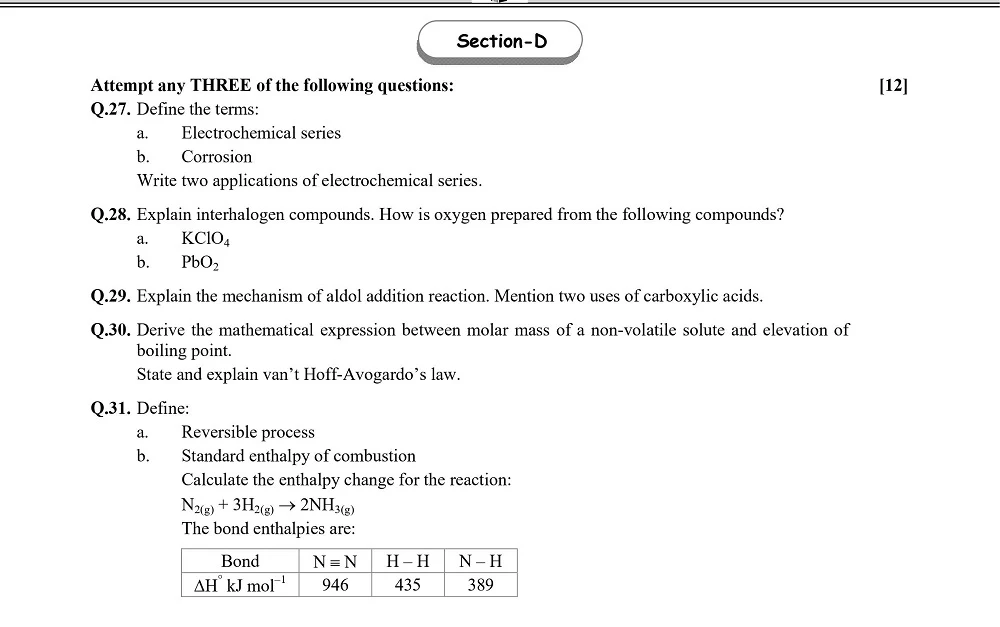
Board 11th class chemistry question papers play a pivotal role in the academic journey of students. They serve as a crucial tool for self-assessment, aiding students in identifying their strengths and areas that require improvement.
Understanding the Structure of Chemistry Question Papers
➭ Format of 11th class chemistry question papers
Before diving into preparation, it's essential to understand the format of the question papers. This section will provide insights into the structure, including the number of sections, types of questions, and marking schemes.
➭ Distribution of marks across different sections
Understanding the distribution of marks can guide students in allocating their time wisely during the exam. We'll explore how different sections contribute to the overall score.
Significance of Practicing Past Papers
➭ Familiarizing with exam patterns
Practicing past papers allows students to become familiar with the recurring patterns in exams. This familiarity reduces anxiety and boosts confidence levels during the actual test.
➭ Identifying important topics and trends
By regularly practicing past papers, students can identify the topics that frequently appear in exams. This insight helps them prioritize their study materials and focus on areas with higher weightage.
How to Approach Different Question Types
➭ Multiple-choice questions
Multiple-choice questions are a common format in chemistry exams. This section provides tips on approaching and mastering this question type.
➭ Short answer questions
Short answer questions require concise yet comprehensive responses. Strategies for effectively tackling these questions will be discussed.
➭ Long-form and essay-type questions
For questions demanding longer responses, a specific approach is necessary. This section guides students on structuring and presenting their answers effectively.
Chemistry Handwritten Short Notes 📚 for Class 11 & 12 | Free PDF Downloads
11th class Chemistry - Resources & Key Concepts
➭ Matter, states of matter, and their properties
➭ Laws of chemical combination
➭ Dalton's atomic theory
➭ Mole concept and stoichiometry
➭ Chemical equations and balancing
➭ Subatomic particles: protons, neutrons, electrons
➭ Atomic number, mass number, isotopes
➭ Atomic models: Rutherford, Bohr, modern quantum mechanical model
➭ Electronic configuration and Aufbau principle
➭ Periodic trends: ionization enthalpy, electron affinity, atomic size
➭ Modern periodic table and its organization
➭ Classification of elements into metals, non-metals, and metalloids
➭ Periodic trends in physical and chemical properties
➭ Ionic bonding: formation, characteristics, examples
➭ Covalent bonding: Lewis structure, octet rule, types of covalent bonds (single, double, triple)
➭ Coordinate covalent bonding
➭ Metallic bonding
➭ VSEPR theory and shapes of molecules
➭ Gaseous state: kinetic theory of gases, ideal gas equation, gas laws
➭ Liquid state: properties of liquids, intermolecular forces (hydrogen bonding, dipole-dipole interactions, London dispersion forces)
➭ Solid state: crystalline and amorphous solids, types of crystals, unit cell
➭ System, surroundings, types of systems (open, closed, isolated)
➭ First law of thermodynamics: internal energy, work, heat
➭ Enthalpy (H) and its calculations
➭ Hess's law of constant heat summation
➭ Second law of thermodynamics: entropy (S), spontaneity of reactions, Gibbs free energy (G)
➭ Reversible and irreversible reactions
➭ Chemical equilibrium: dynamic equilibrium, equilibrium constant (Kp andKc)
➭ Factors affecting equilibrium (concentration, temperature, pressure)
➭ Le Chatelier's principle
➭ Oxidation and reduction, redox reactions
➭ Balancing redox reactions by oxidation number method
➭ Types of redox reactions (combination, decomposition, displacement)
➭ Electrochemical cells: galvanic and electrolytic cells
➭ Electronic configuration trends
➭ Physical and chemical properties of each element
➭ Important compounds and their applications (e.g., sodium chloride, calcium carbonate)
➭ Diagonal relationships between Li and Mg, Be and Al
➭ Electronic configuration trends for each group
➭ Physical and chemical properties of each element group (e.g., Group 13 - Boron and Aluminum, Group 14 - Carbon and Silicon, Group 15 - Nitrogen and Phosphorus)
➭ Important compounds and their applications (e.g., boric acid, ammonia, sulfuric acid)
➭ Allotropy (e.g., carbon as diamond and graphite)
➭ Catenation (ability to form long chains)
Chapter 11: Organic Chemistry - I
➭ Basic concepts of organic chemistry (bonds, functional groups)
➭ Hydrocarbons: alkanes, alkenes, alkynes, aromatic hydrocarbons
➭ Isomerism (structural, geometrical, optical)
➭ Nomenclature of organic compounds (IUPAC system)
➭ Reactions of alkanes (substitution, combustion)
Chapter 12: Organic Chemistry - II
➭ Alcohols, phenols, ethers
➭ Aldehydes, ketones, carboxylic acids
➭ Amines, amides
➭ Organic compounds in everyday life (drugs, polymers, dyes)
Chapter 13: Polymers
➭ Classification of polymers (addition, condensation)
➭ Natural and synthetic polymers
➭ Important polymers and their properties (e.g., polyethylene, nylon, polyester)
➭ Biodegradable polymers and environmental concerns
Chapter 14: Environmental Chemistry
➭ Environmental pollution: types, sources, effects
➭ Air pollution, water pollution, soil pollution
➭ Strategies for pollution control and waste management
➭ Green chemistry and sustainable development
Chemistry Question Past Papers
FAQs:
How often should I practice 11th class chemistry question papers?
Practicing question papers regularly, ideally once a week, is recommended for optimal results.
Are online platforms reliable sources for accessing question papers?
Yes, many reputable educational platforms provide reliable access to a variety of question papers.
How can I effectively manage time during the exam?
Allocate specific time limits for each section and practice sticking to them during mock exams.
What should I do if I consistently make the same mistakes in practice papers?
Identify the pattern of mistakes, seek additional resources for those topics, and consult with teachers for clarification.
Where can I find tips from successful students?
Online forums, educational websites, and interviews with toppers often share valuable insights and strategies.