States of Matter - The fundamental unit of matter is the atom. Atoms are grouped together by chemical bonds to form molecules. The arrangement and motion of these molecules determine the physical state of matter, which can be classified into three main categories: solids, liquids, and gases.
States of Matter - Solids
Properties: Solids have a definite shape and volume. They are rigid and resist deformation. The particles (atoms or molecules) in solids are tightly packed and held together by strong intermolecular forces. These forces restrict the movement of the particles, giving solids their fixed shape and volume.
States of Matter - Liquids
Properties: Liquids have a definite volume but no definite shape. They take the shape of the container they are in. The particles in liquids are closer together than in gases but further apart than in solids. The intermolecular forces are weaker than in solids but stronger than in gases. This allows the particles to move more freely, giving liquids their fluidity.
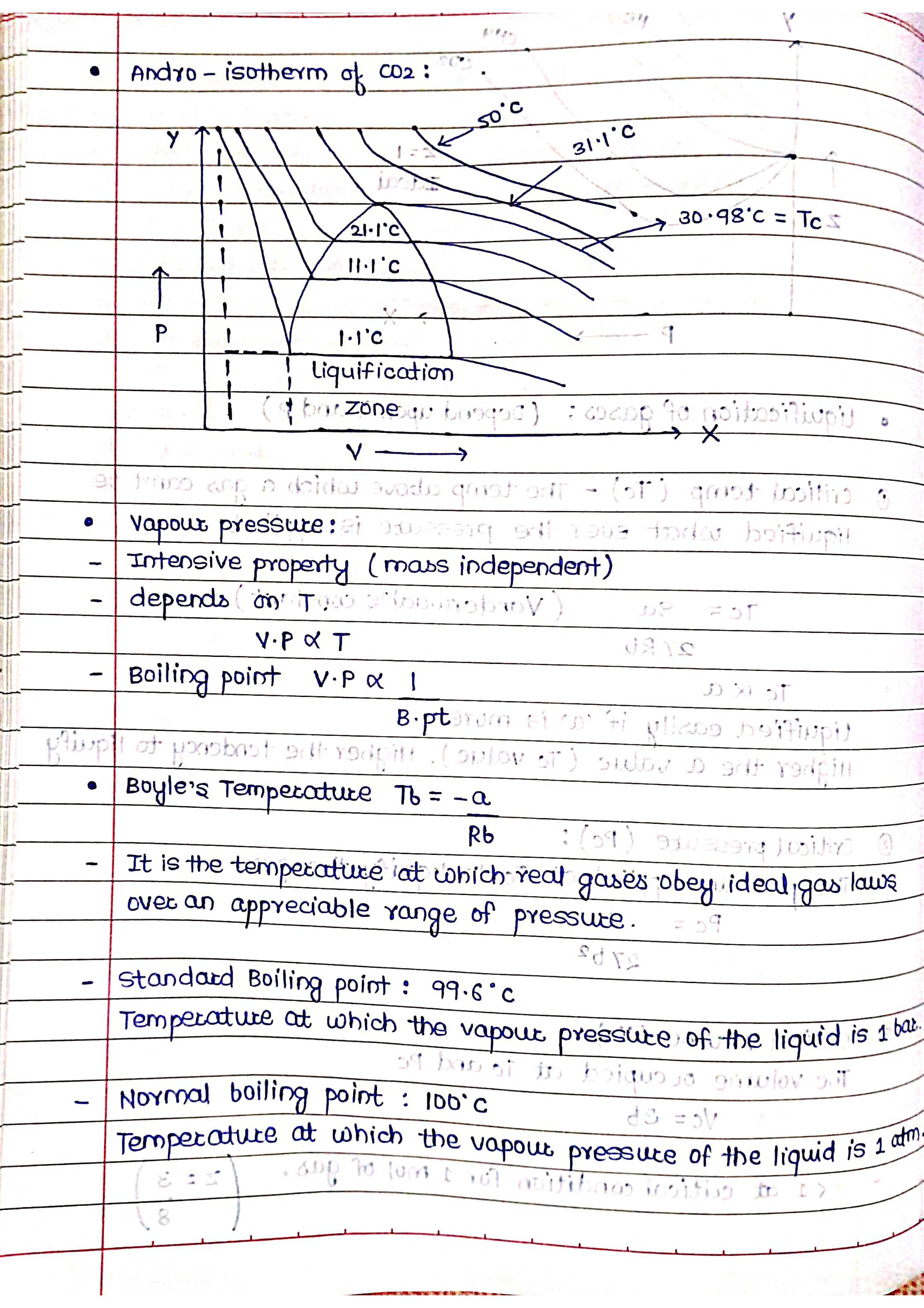
States of Matter - Gases
Properties: Gases have neither a definite shape nor a definite volume. They expand to fill the container they are in. The particles in gases are far apart and have very weak intermolecular forces. This allows the particles to move freely and independently in all directions. Gases are highly compressible due to the large spaces between the particles.
States of Matter - Plasma
In addition to the three main states of matter, plasma is sometimes considered a fourth state of matter. Plasma is a hot, ionized gas in which some or all of the atoms have been stripped of their electrons. Plasma is often found in stars and lightning.
States of Matter - Phase Changes
Matter can change from one state to another by adding or removing heat. The addition of heat increases the kinetic energy of the particles, which can overcome the intermolecular forces and cause the matter to change from a solid to a liquid (melting), from a liquid to a gas (evaporation), or from a gas to a plasma. Conversely, the removal of heat decreases the kinetic energy of the particles and can cause the matter to change from a gas to a liquid (condensation), from a liquid to a solid (freezing), or from a plasma to a gas.
Importance of States of Matter
The understanding of states of matter is essential in many fields of chemistry, physics, and biology. For example, the properties of solids, liquids, and gases are important in the design of materials, the development of new technologies, and the understanding of biological processes.
Chemistry Short Notes 📚⌛
1. Some Basic Concepts of Chemistry Short Notes 📚
2. Atomic Structure — Chemistry Short Notes 📚
3. Periodic table — Chemistry Short Notes 📚
4. Chemical Bonding — Chemistry Short Notes 📚
5. P-Block Elements 1 - Chemistry Short Notes 📚
6. Thermodynamics — Chemistry Short Notes 📚
7. Chemical Equilibrium — Chemistry Short Notes 📚
8. Ionic Equilibrium — Chemistry Short Notes 📚
9. Redox Reaction — Chemistry Short Notes 📚
10. Hydrogen — Chemistry Short Notes 📚
11. S-Block Elements - Chemistry Short Notes 📚