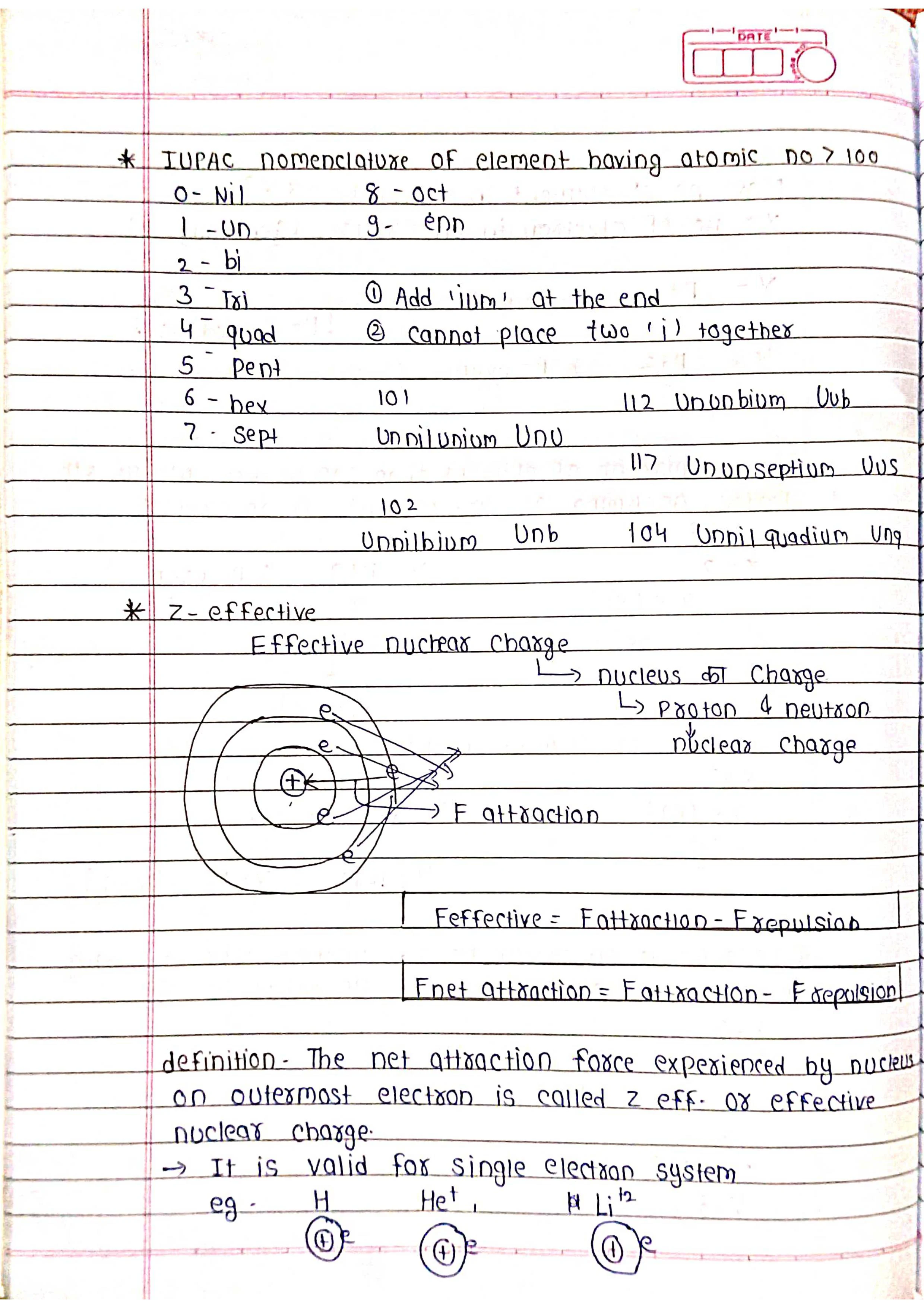
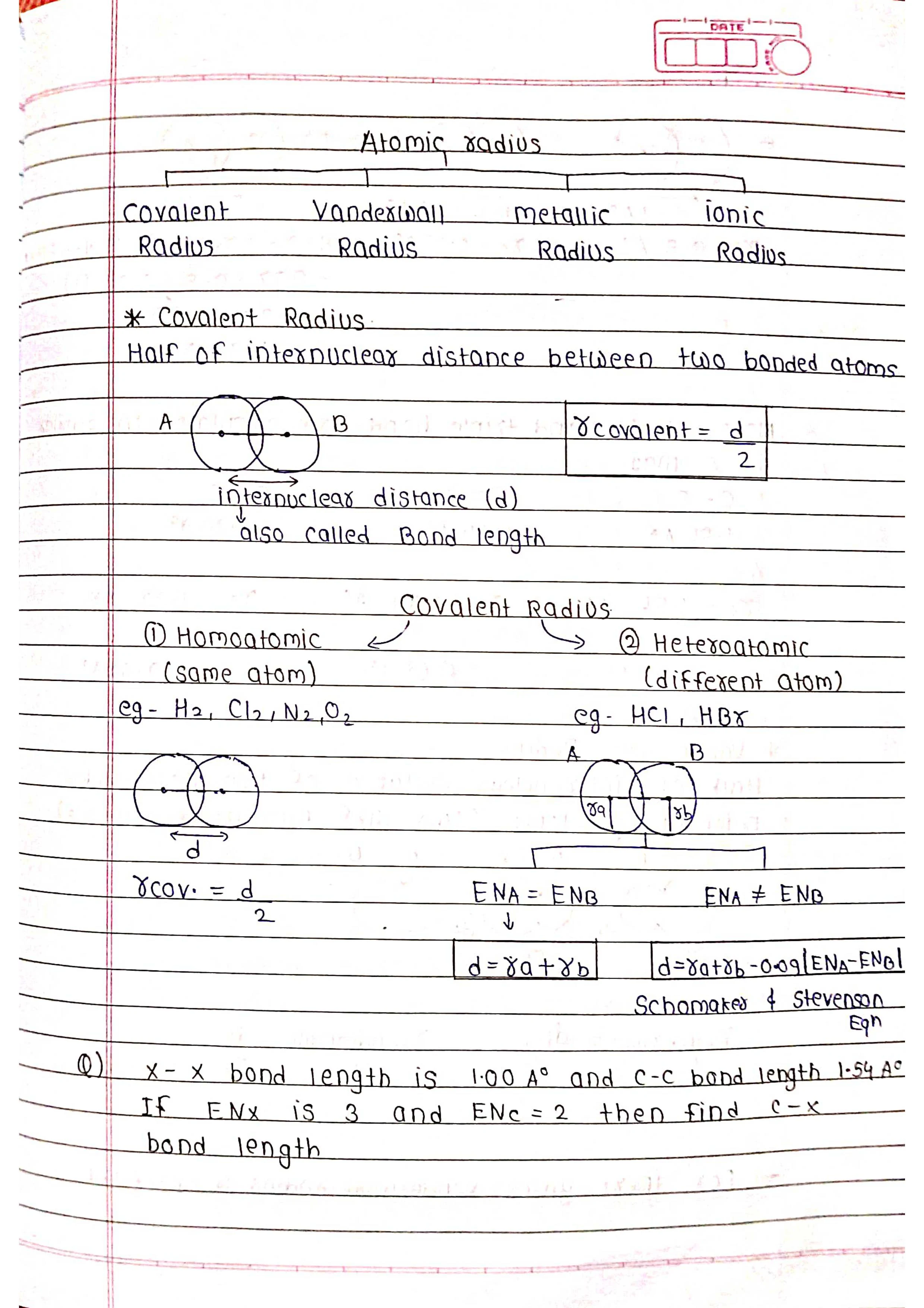
The periodic table is one of the most important tools in chemistry. It's a chart that organizes all the known chemical elements in a way that reveals their relationships and properties.
Here are the key things to know about the periodic table for your chemistry short notes:
Periodic Table - Organization
➡️ Elements are arranged in rows (periods) and columns (groups).
➡️ Elements are ordered by atomic number, which is the number of protons in the nucleus of an atom.
➡️ Horizontal rows are called periods. There are 7 periods in the current periodic table.
➡️ Vertical columns are called groups. There are 18 groups in the periodic table.
![Periodic Table - Chemistry Short Handwritten Notes [PDF]📚 Periodic Table - Chemistry Short Handwritten Notes [PDF]📚](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhR-7lqdQFXCN82tqiaEKLIvWDeNalVOzIAZ_7RZLRHGSGBhhomHADz9iwGXu8qs1gKeGqwy0YCP4Op0dYR38hTHJI72GfUXboG79DCEsd5k5iu5BFxQpFoji8x9p-ePjETDarnOsy3mDorp699oSAzIUdTFvHRGcUrMSmsuTYgeGrabS6T6OnYlYS-bd0/s16000-rw/Periodic%20Table%20-%20Chemistry%20Short%20Handwritten%20Notes.jpeg)
Periodic Table - Key Concept
➡️ Atomic number: The number of protons in an atom's nucleus, determining its identity as an element and its place in the table.
➡️ Electron configuration: The arrangement of electrons in an atom's orbitals, influencing its chemical behavior.
➡️ Periodic trends: Properties like atomic size, ionization energy, electronegativity, and metallic character exhibit predictable patterns across the table.
Periodic Table - Groups
1. Group members share similar properties due to having the same number of valence electrons (outermost electrons involved in bonding).
2. Some important groups include:
➭Group 1 (Alkali metals): highly reactive metals, readily forming ionic bonds.
➭ Group 17 (Halogens): non-metals forming diatomic molecules and ionic bonds.
➭Group 18 (Noble gases): unreactive monatomic gases with filled valence orbitals.
Periodic Table - Periods
1. As you move left to right across a period, atomic number and effective nuclear charge increase, leading to:
➭Decreasing atomic size.
➭Increasing ionization energy.
➭Increasing electronegativity.
➭Transition from metals to non-metals.
2. Moving down a group, atomic size and metallic character generally increase.
Periodic Table - Trends
➭As you move down a group, the elements become more metallic. This means they are more likely to lose electrons and form positive ions.
➭As you move across a period, the elements become more nonmetallic. This means they are more likely to gain electrons and form negative ions.
➭Elements in the same group have similar chemical properties.
Periodic Table - Regions
➭Metals: Located on the left side of the table and most of the center. They are malleable, ductile, and good conductors of heat and electricity.
➭Nonmetals: Located on the right side of the table. They are brittle, poor conductors of heat and electricity, and tend to gain electrons in chemical reactions.
➭Metalloids: Located along the "staircase" line between metals and nonmetals. They have properties of both metals and nonmetals.
➭Noble gases: Located in the far right column. They are unreactive gases because their outer electron shells are full.


![Periodic Table - Chemistry Short Handwritten Notes [PDF]📚 Periodic Table - Chemistry Short Handwritten Notes [PDF]📚](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhR-7lqdQFXCN82tqiaEKLIvWDeNalVOzIAZ_7RZLRHGSGBhhomHADz9iwGXu8qs1gKeGqwy0YCP4Op0dYR38hTHJI72GfUXboG79DCEsd5k5iu5BFxQpFoji8x9p-ePjETDarnOsy3mDorp699oSAzIUdTFvHRGcUrMSmsuTYgeGrabS6T6OnYlYS-bd0/s16000-rw/Periodic%20Table%20-%20Chemistry%20Short%20Handwritten%20Notes.jpeg)

![Periodic Table - Chemistry Short Handwritten Notes [PDF]📚 Periodic Table - Chemistry Short Handwritten Notes [PDF]📚](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEiPvPgAQ-2gN-glu8tMvmByJG60Wn6TRxxAAj5OVBgfwdAewgUPKvZSVyJLP7naRKLXbleLyzJhjRehSRXcCRxyClDsCI4QFWXXOePRAoS_tFnF2-3EdT4qj10rLdV66ZSOWcSRvlOdA2ZwJQJeBkSwgm-kKFniYDDkZQp4yLErcEe8A-1Qo4Hr1hE9Lo0/s16000-rw/Periodic%20Table%20of%20Elements.jpg)
![Periodic Table - Chemistry Short Handwritten Notes [PDF]📚 Periodic Table - Chemistry Short Handwritten Notes [PDF]📚](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEivv4mYytUW4Lw50BeqNtG9LVmtwRF9fHOPMGyETiyIkkamuc-9vKdKKLUCnAtrjhHUWFpo4VhA4kyfrwZX5_VxHWUHamMQrJBRLTvGuDCH-3uOSxS9GR9iuZPYh9kxpmNVYVlzB3rhrxpZ9ZURzvPnMC7F8BKKyR1ihCgtIZK7OSf02s-S5fzx9U-RMLU/s16000-rw/Periodic%20Table%20-%20Chemistry%20Short%20Handwritten%20Notes%202.jpeg)